Preface
The following material represents a simplified version of the nervous system. This is by design, since the beginning student can benefit from such a simplification. On the other hand, it is intended that the facts presented here are true, accurate and clinically functional. Those students who master this material should have little difficulty adapting to more complex views of the nervous system during future studies. In addition to providing a "bird's eye" view of the nervous system, this work will attempt to demonstrate analogies which may assist the student in remembering the complex functions which are contact herein. It is hoped that the student will have fun mastering this material. Enjoyment is the key to enlightenment.

Introduction:
What makes the nervous system unique is that it is made up of a network of interconnected electrically active cells called neurons. These cells endow the nervous system with the capability to form complex electrical pathways and to transport this integrated information throughout the body. Most of the cells interact through substances released from their synaptic terminals and these chemicals alter the membrane potentials of a variety of post-synaptic cells (including other neurons, skeletal muscles, smooth muscles, cardiac muscles and glands). The chemical transmission of neurons results in alteration in transmembrane ion channels. This results in ion movements across membranes, leading to alterations in the activities of the post-synaptic cell.
The central nervous system receives information about the external environment, using complex behaviors to allow the animal to negotiate this environment. This function is carried out by the somatic nervous system which regulates skeletal muscle activity. In addition, the central nervous system listens to the internal workings of the body and regulates those function which maintain the animal's health. These functions are performed by the autonomic nervous system. These two basic activities of the central nervous system are not completely separated, since the integration of the whole organism is carried out seamlessly.
The central nervous system functions in concert with the body. Disease of many other body parts can affect the nervous system and disease of the nervous system can result in dysfunction of other parts of the body. While this may appear to lead to undue difficulties in understanding the relationship between the central nervous system and the rest of the body, it also provides the challenge by which neurology becomes an exciting clinical discipline. The diversity by which neurologic disease can manifest makes neurology an interesting field of endeavor.
Cells of the Nervous System:
Neurons. One of the important distinguishing cells of the nervous system is the neuron. It is the electrically active cell and is responsible for the majority of the functional properties which we ascribe to the nervous system. Neurons come in three basic types: 1) the (pseudo)-unipolar cells; 2) bipolar cells, and 3) multipolar cells. The majority of the neurons of the nervous system are of the latter cell type. The gray matter of the central nervous system is largely made up of neuronal cell bodies and the bodies of their supportive cells. When neuronal cell bodies are accumulated within the central nervous system, the resultant gray matter is called a nucleus. A group of neuronal cell bodies in the peripheral nervous system is called a ganglion.
 Unipolar neurons
are specialized neurons which make up the majority of sensory neurons from
the body and head. They have a distal process and a proximal process, both
of which function as axons. The cell body of these neurons lie in the dorsal
root ganglia and the sensory ganglia of the cranial nerves which innervate
skeletal muscle. The distal process begins at a specialized sensory organ,
which transduces information which initiates an action potential which
propagates toward the cell body. This action potential does not degrade,
since the distal process is an axon in character. The action potential
reaches the proximal axonal process and continues toward the central nervous
system unabated. The proximal axon terminates in the spinal cord or in
nuclei in the brainstem, hence it is sometimes called the central process
of the unipolar neuron.
Unipolar neurons
are specialized neurons which make up the majority of sensory neurons from
the body and head. They have a distal process and a proximal process, both
of which function as axons. The cell body of these neurons lie in the dorsal
root ganglia and the sensory ganglia of the cranial nerves which innervate
skeletal muscle. The distal process begins at a specialized sensory organ,
which transduces information which initiates an action potential which
propagates toward the cell body. This action potential does not degrade,
since the distal process is an axon in character. The action potential
reaches the proximal axonal process and continues toward the central nervous
system unabated. The proximal axon terminates in the spinal cord or in
nuclei in the brainstem, hence it is sometimes called the central process
of the unipolar neuron.
 Bipolar cells are
specialized sensory neurons for the transmission of special senses. As
such, they are part of the sensory pathways for smell, sight, taste, hearing
and vestibular functions. Bipolar neurons have a single dendrite and axon,
separated by the cell body (soma) of the neuron.
Bipolar cells are
specialized sensory neurons for the transmission of special senses. As
such, they are part of the sensory pathways for smell, sight, taste, hearing
and vestibular functions. Bipolar neurons have a single dendrite and axon,
separated by the cell body (soma) of the neuron.
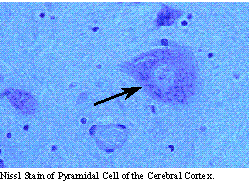
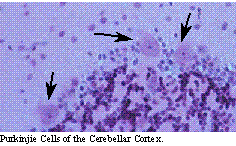
Multipolar neurons make up the majority of the neurons of the nervous system. They process a large dendritic tree and a single axon, which may travel long distances to connect other regions of the nervous system. These are the typical cells of the cerebral cortex, cerebellum, brain stem and spinal cord. The brain stem and spinal cord give rise to motor neurons which leave the central nervous system, enter the peripheral nervous system and innervate the muscles, viscera and glands of the rest of the body.
Glia. The main supportive tissues of the nervous system is composed of glial cells. Glial cells provide nutritive functions toward neurons, help maintain the blood-brain barrier and act as the primary cellular defense mechanism for the central nervous system. The four types of glial cells are the oligodendroglial cells, the astrocytes, the microglia and the ependymal cells. In the peripheral nervous system, oligodendroglial cells are called schwann cells.
 The oligodendroglial
and schwann cells are the myelin producing cells in the nervous system.
By wrapping the cell membranes around axonal processes, myelination is
accomplished. One schwann cell myelinates a single axon, while, in the
central nervous system, a single oligodendroglial cell may myelinate several
axonal processes. Myelination of axons by oligodendroglial and schwann
cells insulates axons and improves the speed of action potential propagation
by neurons. Besides myelination, these cells provide local nutritional
support for the axonal processes which they contact. They also help inactivate
neurotransmitters substances and may play a role in short-term memory.
The oligodendroglial
and schwann cells are the myelin producing cells in the nervous system.
By wrapping the cell membranes around axonal processes, myelination is
accomplished. One schwann cell myelinates a single axon, while, in the
central nervous system, a single oligodendroglial cell may myelinate several
axonal processes. Myelination of axons by oligodendroglial and schwann
cells insulates axons and improves the speed of action potential propagation
by neurons. Besides myelination, these cells provide local nutritional
support for the axonal processes which they contact. They also help inactivate
neurotransmitters substances and may play a role in short-term memory.
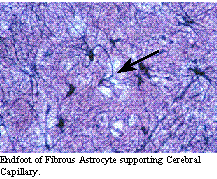
The astrocyte provides the scaffolding maintaining the structure of the nervous system. They play an important role in isolating the nervous system from the rest of the body by re-enforcing the structural barrier and providing an enzymatic barrier which helps prevent entry of materials into the nervous system. Astrocytic endfeet surround the capillaries of the nervous system, supporting the tight junctions of the capillaries in the nervous system and are an important part of the blood-brain barrier.
The microglial cells are the primary cellular defense mechanism in the nervous system. They are phagocytic and can help digest infectious agents and damaged neural tissue. In response to infection, inflammation or tissue damage, these cells proliferate and accumulate in the parenchyma of the nervous system. Their levels will also increase in the cerebral spinal fluid.
Ependymal cells line the ventricular surfaces and the central canal of the spinal cord. They help provide part of the barrier between the nervous tissue and the cerebral spinal fluid. In the lateral, third and forth ventricles, the ependymal cells along with capillaries forms the choroid plexus, producing 70% of the cerebral spinal fluid which circulates in and around the nervous tissues.
Anatomy of an Action Potential:
 One of the most
important properties of the nervous system, which helps define it, is its
ability to produce and propagate action potentials. The resting membrane
potential of most neurons is at around -70 mV. This membrane potential
is maintained by the facts that the membrane itself is semipermeable, selectively
regulating the movement of charged materials through it, and by the presence
of an ATP-dependent sodium and potassium pump. This energy-dependent ion
pump actively exchanges internal sodium ions for external potassium ions,
even against their concentrations gradients. As such, the sodium content
is greater in the extracellular fluid then the intracellular fluid of neurons.
In addition, potassium ion concentration is greater inside than outside
neurons. Due to the presence of negatively-charged, intracellular organic
acids, the concentration of chloride is less in the intracellular fluid
than outside the neuron, as well. The net effect of the positive and negative
concentration and electrostatic forces is that the neuron is negatively
charged compared to the extracellular fluid.
One of the most
important properties of the nervous system, which helps define it, is its
ability to produce and propagate action potentials. The resting membrane
potential of most neurons is at around -70 mV. This membrane potential
is maintained by the facts that the membrane itself is semipermeable, selectively
regulating the movement of charged materials through it, and by the presence
of an ATP-dependent sodium and potassium pump. This energy-dependent ion
pump actively exchanges internal sodium ions for external potassium ions,
even against their concentrations gradients. As such, the sodium content
is greater in the extracellular fluid then the intracellular fluid of neurons.
In addition, potassium ion concentration is greater inside than outside
neurons. Due to the presence of negatively-charged, intracellular organic
acids, the concentration of chloride is less in the intracellular fluid
than outside the neuron, as well. The net effect of the positive and negative
concentration and electrostatic forces is that the neuron is negatively
charged compared to the extracellular fluid.
Neurons also possess specialized transmembrane channels which allow for selective passage of ions across the membrane. Many of these channels are electrically gated and are often coupled to certain membrane proteins which act as receptors for neurotransmitter substances. As such, when the receptor is stimulated, or the surrounding electrical potential reaches a certain point, the channel opens and allows the flow of ions based upon their concentration gradients. Depending upon the nature of the receptor which has been activated and upon which pore is opened, various ions can cross the membrane, changing the membrane potential. Movement of sodium ions into the neuron results in depolarization of the membrane potential and the membrane potential becomes more positive. Influx of chloride ions hyperpolarizes the neuronal membrane, while efflux of potassium ions, which usually follows the influx of sodium ions, results in repolarization toward the resting potential.
The nature of the cell body and dendrites of neurons is that there is summation of the many neuronal stimuli by the various movements of ions. These summations have both spatial (regional) and temporal (timing) properties. It is the combinations of EPSP (excitatory post-synaptic potentials) and IPSP (inhibitory post-synaptic potentials) which determine the membrane potential of the neuron at any given time. Most EPSP signals are mediated through influx of sodium ions, whereas most IPSP are mediated through chloride ion influx. When the influx of sodium ions exceeds that of chloride to a certain point, the neuronal membrane undergoes rapid depolarization with even more sodium influx. At this point, the membrane potential becomes positive relative to the external fluid and an action potential develops. It spreads over the surface of the dendrites and cell bodies, finally dissipating. The membrane in the wake of the influx of sodium ions alters its permeability to sodium and increases permeability to potassium ions, which exit into the extracellular space. This results in repolarization of the membrane to a negative state, slightly more negative when the membrane is less responsive to additional stimuli, the refractory period. The sodium/potassium pump then re-establishes the normal sodium-potassium balance across the membrane. While the movement of ions is small, it is sufficient to account for the events described.
The axon of neurons is unique in structure and function. The functional difference lies in that the axon propagates action potentials which do not diminish over distance. As such, once the neuronal membrane at the beginning of the axon, the axonal hillock, reaches sufficient depolarization to initiate an action potential, this action potential will continue unabated down the length of the axon until it depolarizes its synaptic terminal. At the nerve terminus, the influx of sodium results in the influx of calcium ions, as well. Here, the calcium ions activate an actin-myosin ratchet which, in turn, pulls synaptic vesicles (containing neurotransmitter substances) to the synaptic cleft. The vesicles fuse with the nerve terminal membrane extruding neurotransmitter substance into the synaptic cleft by an exocytotic process. The neurotransmitter diffuses across the synaptic cleft to interact with receptors on the post-synaptic membrane, changing the post-synaptic membrane's permeability to ions. Thus information is transmitted long distances in the nervous system and past on to other neurons or target organs through ion movements and action potentials.
Mammalian nervous systems developed specialized processes to enable the rapid propagation of action potentials, called myelination. In the central nervous system, oligodendroglial cells and, in the peripheral nervous system, schwann cells wrap around axons. This wrapping, along with the extrusion of the cytoplasm of the myelin-forming cell, leads to the application of many layers of phospholipid membranes (called myelin) around the axon. This insulates the axon, nourishes the axon, and provide greater resistance to ion movements in the area of myelin. The axon modifies to concentrate its ion channels between regions of myelin (the Nodes of Ranvier). Due to the greater capacitance where myelin exists, action potentials propagate down myelinated axons by jumping from node to node. As such, the speed of action potential propagation is greatly enhanced, allowing smaller axons to propagate action potentials with great speed. Without myelination, the mammalian nervous system would have to be many times greater in size (to accommodate much larger axons, whereby maintaining speed of neural propagation) or movement would be markedly slower.
Neural Energy and Blood Flow:
The brain makes up about 2 percent of the body's weight, yet receives 15 percent of the cardiac output and consumes 20 percent of the body's available oxygen. Since the central nervous system has virtually no oxygen storage capacity, it is absolutely dependent on a continuous, uninterrupted supply of oxygen from the cerebral circulation. Within seconds of cessation of blood flowing to the brain, consciousness is lost and irreversible damage to neural tissues will occur in minutes. One of the important functional properties of cerebral circulation is its ability to maintain a constant blood flow over a wide range of system blood pressure. This ability to autoregulate cerebral blood flow by changing the diameter of cerebral arteries and arterioles is controlled primarily in response to changes in the partial pressure of CO2 in blood. In addition to CO2, secondary control of cerebral blood flow is under regulation by sensing the partial pressure of O2 and by neural regulation by catecholinergic neuron which innervate cerebral blood vessels.
In addition to providing a constant supply of oxygen, cerebral blood flow also provides adequate supply of the major energy source used by the central nervous system, glucose. Neural tissue is dependent upon aerobic glycolysis. When either oxygen or glucose are deprived from the nervous system, lactic acid builds up within the tissue and is one of the factors which results in structure damage following interruption of blood supply to the brain. The nervous system, due to the presence of a carrier-mediated system to capture glucose from blood is very efficient in regulating glucose delivery to the brain. The only other natural substances which can substitute for up to 60 percent of the nervous systems glucose requirements are ketone bodies.
Small amounts of energy goes to the production of proteins and neurotransmitter substances. As expected, most of the energy utilized by the central nervous system is used to maintain the sodium/potassium pump to support maintenance of the electrical properties of neurons.
Blood Brain Barrier and CSF Formation:
The central nervous system requires constant environmental conditions in order to function properly. As such, the central nervous system developed mechanisms to maintain this environment and ensure that damaging materials can be excluded, termed the blood-brain barrier. This barrier is both structural and functional. The structural parts of this barrier is based upon the fact that the endothelial cells of the capillaries in the central nervous system have tight junctions, that is the endothelial cell fenestrations, unlike most capillary beds, are exceedingly close together. This prevents all but extremely small particles from passing through the endothelial cells of the central nervous system. In addition to the tight junctions of the endothelial cells, the blood vessels of the central nervous system are reinforced by the presence of astrocytic endfeet, projections of fibrous astrocytes which surround capillaries and provide additional structural support. The addition of endothelial and astrocytic membranes to the barrier increases the membrane phospholipid content in the barrier, preventing passage of materials which are not lypophilic. The functional nature of the blood-brain barrier is due to the presence of enzymatic systems present within the endothelial cells and the astrocytes which form the structural barrier. Materials which nature wants to exclude from entering the central nervous system are broken down by these enzymes into non-threatening materials.
The blood-brain barrier isolates the central nervous system from the rest of the body, protecting the delicate environment necessary for electrochemical transmission of information. Unfortunately, while this barrier helps prevent many diseases from entering the central nervous system, it also reduces the ability of the body's peripheral immune responses from fighting disease within the central nervous system. This is also a formidable barrier to drugs which can be used to treat central nervous system disorders, limiting these drugs to relatively small, non-polar materials. This must be taken into account whenever treating central nervous system diseases with drugs.
In the presence of this formidable barrier, it is a wonder that exchange occurs between the central nervous system and the blood. There is, however, a constant exchange of materials across the blood-brain barrier, leading to the formation of the extracellular fluid in the brain and of the cerebrospinal fluid surrounding the brain. Some of the mechanisms which are involved in the formation of central nervous system fluid are important. The electrolyte content of brain fluids is maintained through an active transport system, so that the chemical composition of these fluids is not that of blood. In addition, there are a number of specialized carrier-mediated transport systems which do not require energy, yet effectively maintain the internal brain environment. An example of this is the carrier-mediated transport system for glucose. A specialized glucose receptor is present in the capillaries of the central nervous system. This molecule combines readily with glucose and in the process of binding, transports and releases glucose across the membrane. This molecule then regenerates so that it can again pick up another glucose molecule from the blood stream. While this is a saturable process, carrier-mediate transport is highly effective at retrieving and delivering materials to the central nervous system.
Cerebrospinal fluid is formed by the choroid plexus contained in the lateral, third and fourth ventricles. This accounts for 70 percent of the cerebrospinal fluid. The remainder is added by diffusion of extracellular fluid into the cerebrospinal fluid across the pial-cerebrospinal fluid barrier. As such, cerebrospinal fluid is formed is the lateral ventricles, passes into the third ventricle by way of the foramen of Monroe. In the third ventricle more fluid is formed which passes by way of the cerebral aqueduct into the fourth ventricle. Additional cerebrospinal fluid is formed in the forth ventricle and proceeds down the central canal of the spinal cord or exits the ventricular system through the foramen of Luschka, caudal to the middle cerebellar peduncle. Cerebrospinal fluid then travels up around the surface of the brain and down the spinal column in the subarachnoid space. Cerebrospinal fluid is then eliminated by pressure sensitive evaginations of the arachnoid into the venous sinuses of the central nervous system. These processes are call the arachnoid granulations. Most of the cerebrospinal fluid is eliminated through the arachnoid granulation, but a small amount is removed where the cranial and spinal nerves exit the dura mater by a process similar to Virchow's space in the eyes.
Cerebrospinal fluid functions as a waste sink, diluting extracellular metabolic products as they are eliminated. It also functions to cushion the central nervous system, protecting the brain in a hydrodynamic system. This allows the central nervous system to accelerate and decelerate without producing jarring pressures on movement. Cerebrospinal fluid also acts as a form of chemical communication between brain regions which are close to the ventricular system. In these regions, neurotransmitter substances are released into the cerebrospinal fluid, transported by cerebrospinal fluid movement to other regions, and the neurotransmitter substances are received by neurons in these regions.
Functional Divisions of the Central Nervous System:
From a simplistic point of view, the central nervous system can be thought of as a truck. It has a drive train (spinal cord) with four wheels (legs) attached. There is a dashboard (brainstem) that controls the headlights (eyes), the air conditioning (skin and lung vessels), and the carburetor (circulatory and digestive systems). There is a transmission (cerebellum) which regulates the speed and rotation of the tires. There is a steering wheel (cerebral cortex) which gives it direction. Finally, there is an exhaust pipe (sacrum) which regulates the emissions.
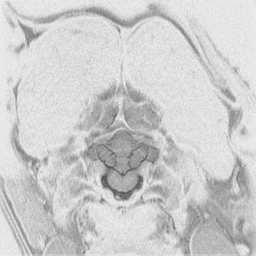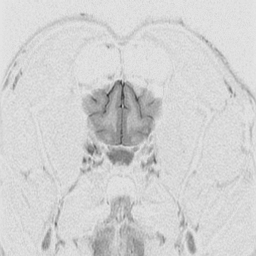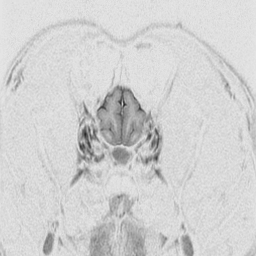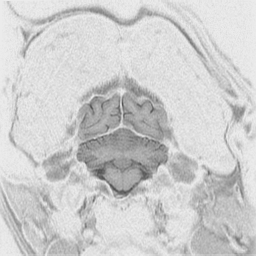 The
cerebral cortex is the behavioral center of the nervous system.
It can be broken into 4 major lobes: frontal, parietal, occipital and temporal.
Each is involved in regulation and integration of behavior. The frontal
lobe is important in motor behavior. The parietal lobe is responsible for
sensory behavior. The occipital lobe regulates visual behavior. The temporal
lobe is responsible for auditory behavior and speech generation. The interaction
of the parts of the cerebral cortex results in the overall behavior of
the animal, resulting in its personality and social patterns. The frontal
lobes, combined with the limbic structures (septal nuclei, dorsal thalamus,
hippocampus, amygdala and hypothalamus) regulates emotional behavior.
The
cerebral cortex is the behavioral center of the nervous system.
It can be broken into 4 major lobes: frontal, parietal, occipital and temporal.
Each is involved in regulation and integration of behavior. The frontal
lobe is important in motor behavior. The parietal lobe is responsible for
sensory behavior. The occipital lobe regulates visual behavior. The temporal
lobe is responsible for auditory behavior and speech generation. The interaction
of the parts of the cerebral cortex results in the overall behavior of
the animal, resulting in its personality and social patterns. The frontal
lobes, combined with the limbic structures (septal nuclei, dorsal thalamus,
hippocampus, amygdala and hypothalamus) regulates emotional behavior.
The diencephalon is made up of the thalamus dorsally and the hypothalamus ventrally. The thalamus is the relay nuclei for all sensory information reaching the cerebral cortex except for olfaction. It is the first place where painful stimuli are perceived by the animal, resulting in a systemic response. The hypothalamus is the "head ganglion" of the autonomic nervous system. Its major function is to maintain internal homeostasis. Through connections with the parasympathetic and sympathetic nervous systems, the hypothalamus regulates heart rate, blood pressure, digestion and elimination. It regulates hunger, thirst, and temperature. Finally, the hypothalamus influences body development and functions by regulating the pituitary gland.
The mesencepahlon consists of the tectum and tegmentum. The tectum of the mesencephalon contains the rostral and caudal colliculli, important in visual and auditory reflexes, respectively. The tegmentum contains the red nucleus which gives rise to the rubrospinal tract which is in turn important for digital flexor control. In addition, the tegmentum contains the motor nuclei for the oculomotor and trochlear nerves. The parasympathetic innervation to the eye begins in nuclei next to the oculomotor nuclei.
The metencephalon consists of the pons ventrally and a specialized motor control system dorsally, the cerebellum. The major distinction of the pons is the presence of the motor nucleus of the trigeminal nerve. The cerebellum coordinates motor movement, ensures that commands initiated in the cerebral cortex are carried out smoothly and is a motor command area for learned, repetitive motor functions. Lesions in the cerebellum result in intention tremors of the head and body, dysmetria and imbalance.
The myelencephalon contains the remainder of the cranial nerve nuclei. The cranial medulla is responsible for vestibular function and balance and facial movements and expression. The caudal medulla is responsible for swallowing, regulation of cardiac and visceral responses and tongue movement.
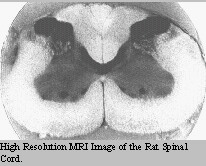 The spinal
cord can be broken into 5 segments: C1-5; C6-T2; T3-L3; L4-S1;
and S1-Cy. Lesions above T2 result in quadriparesis; whereas lesions between
T3 and S1 result in paraparesis. Lesions caudal to S1 result in paralysis
of the anus, urinary bladder and tail. Paraparesis has 2 basic causes.
If the reflexes in the rear legs are present, the lesion is between T3
and L3. If the reflexes are absent, the lesion is between L4 and S1. Quadriparesis
has four causes. If the reflexes are present in all four legs, the lesion
is between C1 and C5. If the reflexes are absent in the front legs, yet
present in the rear legs, the lesion is between C6-T2. If the reflexes
are absent in all four legs, the lesion is a diffuse lower motor neuron
problem. If the rear leg reflexes are present, but the fore legs show signs
of conscious proprioceptive deficits, pain and muscle fasciculations (signs
of "root signature"), then the lesion is between C6-T2. In this case, the
spinal cord is mildly affected from the outside resulting in the rear leg
signs and the spinal nerve root is impinge resulting in the fore leg signs.
Spinal cord diseases tend to affect both sides of the body to some degree,
even though they are often markedly asymmetrical.
The spinal
cord can be broken into 5 segments: C1-5; C6-T2; T3-L3; L4-S1;
and S1-Cy. Lesions above T2 result in quadriparesis; whereas lesions between
T3 and S1 result in paraparesis. Lesions caudal to S1 result in paralysis
of the anus, urinary bladder and tail. Paraparesis has 2 basic causes.
If the reflexes in the rear legs are present, the lesion is between T3
and L3. If the reflexes are absent, the lesion is between L4 and S1. Quadriparesis
has four causes. If the reflexes are present in all four legs, the lesion
is between C1 and C5. If the reflexes are absent in the front legs, yet
present in the rear legs, the lesion is between C6-T2. If the reflexes
are absent in all four legs, the lesion is a diffuse lower motor neuron
problem. If the rear leg reflexes are present, but the fore legs show signs
of conscious proprioceptive deficits, pain and muscle fasciculations (signs
of "root signature"), then the lesion is between C6-T2. In this case, the
spinal cord is mildly affected from the outside resulting in the rear leg
signs and the spinal nerve root is impinge resulting in the fore leg signs.
Spinal cord diseases tend to affect both sides of the body to some degree,
even though they are often markedly asymmetrical.
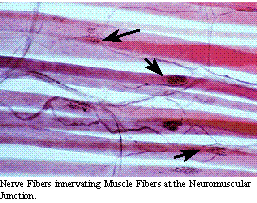 The peripheral
nerves can be affected in pathology. When a single nerve is affected,
the signs of motor and sensory deficits are localized to the specific distribution
of that nerve. Remember that the cranial nerves (unlike the somatic nerves)
usually have the sensory portion of the reflex arc carried in one cranial
nerve, while the motor arm of the reflex is carried in a different cranial
nerve. For example, the papillary light response has the optic nerve carry
the light energy and the oculomotor nerve carries the motor response to
constrict the pupil. Polyradiculopathy can result in quadriparesis with
diminished reflexes in all four legs.
The peripheral
nerves can be affected in pathology. When a single nerve is affected,
the signs of motor and sensory deficits are localized to the specific distribution
of that nerve. Remember that the cranial nerves (unlike the somatic nerves)
usually have the sensory portion of the reflex arc carried in one cranial
nerve, while the motor arm of the reflex is carried in a different cranial
nerve. For example, the papillary light response has the optic nerve carry
the light energy and the oculomotor nerve carries the motor response to
constrict the pupil. Polyradiculopathy can result in quadriparesis with
diminished reflexes in all four legs.
Upper Motor Neurons and Lower Motor Neurons:
The lower motor neuron represents the motor nuclei of the cranial nerves (example motor nucleus of cranial nerve V) and the ventral horn cells (alpha motor neuron) of the spinal cord. The motor unit includes the motor neuron, its axon, the muscle on innervation, the sensory fiber from the muscle and tendon stretch receptors, the central sensory process which feed back upon the motor neuron. The integrity of each of these components of the motor unit is needed to maintain a reflex arc. The lower motor neuron is the final pathway by which motor activity (reflex activity) occurs. If they are damaged, reflexes are lost.
Upper motor neurons are motor pathways which feed information from other parts of the central nervous system upon the lower motor neuron. Upper motor neurons regulate the activity of the lower motor neuron, integrating information to cause lower motor neurons to behave in specific ways. While certain upper motor neurons facilitate lower motor neuron activity, most inhibit. As such, upper motor neuron lesions usually disinhibit lower motor neurons, resulting in increased reflex activity. Upper motor neurons receive information from numerous sources including ascending sensory pathways. These inputs provide feedback upon the upper motor neurons, providing information which alters their regulation of the lower motor neuron. From a practical point of view, upper motor neurons represent the cortical and cerebellar control of motor behavior through modulation of motor pathways residing within the central nervous system. As such, these are a form of internuncial neurons which live within the central nervous system, functioning to alter the activity of lower motor neurons.
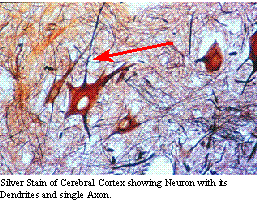 Lower motor neurons
can be thought of as the mechanism of reflexes, while upper motor neurons
are the cause of motor behavior. Since upper motor neurons (or their inputs)
descend the entire neural axis, any lesion of the nervous system results
in an upper motor neuron lesion caudal to the lesion. Lower motor neuron
lesions are limited to those locations where reflexes can be tested; that
is, the cranial nerves and the pectoral and pelvic plexus. While any lesion
will cause local lower motor neuron dysfunction, if the motor neurons do
not innervate a reflex which can be tested, it will go unnoticed. On the
other hand, disruption of the upper motor neuron or its sensory pathways
can be seen by looking at the effects on lower motor neurons caudally.
This disruption is commonly referred to as "long tract signs".
Lower motor neurons
can be thought of as the mechanism of reflexes, while upper motor neurons
are the cause of motor behavior. Since upper motor neurons (or their inputs)
descend the entire neural axis, any lesion of the nervous system results
in an upper motor neuron lesion caudal to the lesion. Lower motor neuron
lesions are limited to those locations where reflexes can be tested; that
is, the cranial nerves and the pectoral and pelvic plexus. While any lesion
will cause local lower motor neuron dysfunction, if the motor neurons do
not innervate a reflex which can be tested, it will go unnoticed. On the
other hand, disruption of the upper motor neuron or its sensory pathways
can be seen by looking at the effects on lower motor neurons caudally.
This disruption is commonly referred to as "long tract signs".
The ability to recognize upper motor neuron and lower motor neuron dysfunctions is the basis for clinical neurology and a key concept in evaluating the nervous system for disease.
Last updated 27 August 2002