PARAPARESIS AND PARAPLEGIA
by R.M. Clemmons, DVM, PhD
Associate Professor of Neurology & Neurosurgery
Introduction:
Parapareis (weakness in the rear limbs) and paraplegia (paralysis of the
rear limbs) unaccompanied by signs of additional CNS disturbance suggests
that the disease is located caudal to T2. If the rear limb reflexes are
intact, the lesion is between T2 and L3. If the rear leg reflexes are diminished
to absent, the lesion is between L4 and S2. This can be refined further
in that lesions between L4 and L5 result in loss of femoral nerve function,
manifested as a decrease in the patellar tendon reflex and inability to
support weight in the rear legs. Lesions between L6 and S2 result in sciatic
nerve dysfunction, reducing rear leg withdrawal, cranial tibialis muscle,
gastrocnemius muscle and sciatic nerve reflexes.
The differential diagnosis of paraparesis and paraplegia include a number
of congenital diseases, including vertebral malformations, various spinal
cord malformations, multiple cartilaginous exostoses, lysosomal storage
diseases, and breed-specific disorders. Other disorders are similar to
those which affect the cervical spinal cord including meningomyelitis (from
various causes), degenerative disc disease, spinal cord trauma, fibrocartilaginous
infarction, and neoplasia. In some breeds, the differential also includes
degenerative myelopathy.
Diagnostic Approach:
 The neurologic
assessment of patients with rear leg problems helps to confirm that the
disease is neurologic in nature and its location. Weakness can indicate
neurologic disease, muscle disease or systemic illness. On the other hand,
reproducible deficits in proprioception usually is indicative of neurologic
disease, whether knuckling, stumbling or falling or conscious proprioceptive
deficits or dysmetria of unconscious proprioceptive dysfunction. When deciding
whether a rear leg lameness is secondary to orthopedic or neurologic disease,
examination of proprioceptive function can help make the differentiation.
The neurologic
assessment of patients with rear leg problems helps to confirm that the
disease is neurologic in nature and its location. Weakness can indicate
neurologic disease, muscle disease or systemic illness. On the other hand,
reproducible deficits in proprioception usually is indicative of neurologic
disease, whether knuckling, stumbling or falling or conscious proprioceptive
deficits or dysmetria of unconscious proprioceptive dysfunction. When deciding
whether a rear leg lameness is secondary to orthopedic or neurologic disease,
examination of proprioceptive function can help make the differentiation.
Unlike cervical disease, there are several neurologic tests which can
assist in lesion localization with TL disease. If the lesion is between
T2 and L3, Schiff-Sherrington syndrome may be seen. Also, between T2 and
L4 is the panniculus response, where superficial stimulation of the skin
over the back results in stimulation of intraspinal pain pathways with
the resultant contraction of the latisimus dorsi muscle. Due to the overlap
of sensory dermatomes, the panniculus response will be absent 1-2 segments
caudal to the lesion. Hyperpathia on deep palpation will be present at
the cranial edge of the lesion and hyperesthesia will be evident on pin
prick of the skin at the cranial and caudal edges of the lesion. By locating
hyperpathia and hyperesthesia and demonstrating the loss of the panniculus
response 1-2 segments caudally, the lesion is found.
The ancillary diagnostic tests for TL spinal disease are identical to
those for cervical disease with the exception that lumbar CSF should be
obtained in most instances. Since the flow of CSF is from cranial to caudal,
lumbar CSF more accurately represents changes within the TL spinal column.
This is usually obtained by carefully passing a needle into the subarachnoid
space between L5-L6 or L4-L5.
Specific Conditions:
Intervertebral Disc Disease:
Intervertebral disc (IVD) disease is a surgical disease. Now, that has
been said I will attempt to explain the disease and why surgery is the
treatment of choice. Not only is IVD disease a common problem, it is one
which I personally like, since it is one neurologic disease which can be
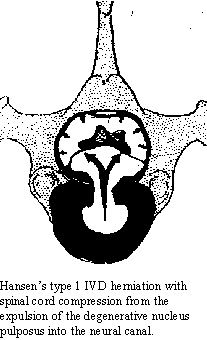
cured. IVD disease can occur as a protrusion of the IVD (Hansen's Type
2 IVD) with the dorsal annulus still covering the disc material or as a
herniation of the nucleus pulposus into the neural canal (Hansen's Type
1 IVD). The former is most common in non-chondrodystrophic animals (straight-legged
dogs) and occurs as a result of age-related changes in the IVD. As animals
age, the water content of the IVD diminishes and the collagen content increases
(similar to nuclear sclerosis of the eye). This results in a decrease in
the IVD elasticity, leading to degeneration of the annulus fibrosis and
protrusion of the IVD. Depending upon the location, this can result in
spinal cord or nerve root compression and development of neurologic signs.
The onset of signs increases with age, peaking around 8-10 years of age.
This type of IVD protrusion is uncommon before 5-6 years of age.
 On the other hand,
chondrodystrophic breeds of dogs are prone to the development of IVD herniation
early in life. In these breeds (including dachshunds, beagles, pekinese,
miniature poodles, cocker spaniels, pomeranians and basset hounds), there
is a metaplasia of the nucleus pulposus whereby the normal collagen fibers
of the nucleus are replaced by hyaline fibers. The hyaline fibers are less
elastic than collagen fibers leading to degeneration of the annulus fibrosis.
The hyaline fibers during this degenerative process calcify, creating further
inelasticity. Due to the fact that the annulus fibrosis is thinnest dorsally
toward the spinal cord, the least line of resistance for the degeneration
and breakdown of the annulus is toward the spinal cord. Ultimately, the
annulus ruptures allowing the herniation of the degenerative nucleus into
the neural canal, compressing the spinal cord. Not only does the IVD material
compress the spinal cord, but the degenerative material is irritative in
nature. The presence of the herniated material in the epidural space causes
inflammation, furthering the swelling associated with the herniation.
On the other hand,
chondrodystrophic breeds of dogs are prone to the development of IVD herniation
early in life. In these breeds (including dachshunds, beagles, pekinese,
miniature poodles, cocker spaniels, pomeranians and basset hounds), there
is a metaplasia of the nucleus pulposus whereby the normal collagen fibers
of the nucleus are replaced by hyaline fibers. The hyaline fibers are less
elastic than collagen fibers leading to degeneration of the annulus fibrosis.
The hyaline fibers during this degenerative process calcify, creating further
inelasticity. Due to the fact that the annulus fibrosis is thinnest dorsally
toward the spinal cord, the least line of resistance for the degeneration
and breakdown of the annulus is toward the spinal cord. Ultimately, the
annulus ruptures allowing the herniation of the degenerative nucleus into
the neural canal, compressing the spinal cord. Not only does the IVD material
compress the spinal cord, but the degenerative material is irritative in
nature. The presence of the herniated material in the epidural space causes
inflammation, furthering the swelling associated with the herniation.
Almost all chondrodystrophic dogs will show some degree of IVD degeneration
within a year of age. The earliest I have seen clinical IVD herniation
is these dogs is at 7 months. Usually the onset is between 2-3 years of
age with the peak incidence being between 4-6 years of age. There are 26
IVD in dogs, any one of which can herniate. However, IVD herniation is
less common in the upper thoracic region due to the conjugal ligament which
connects the rib heads and reinforces the dorsal annulus in that area.
Of the remaining spinal column regions, 20% of IVD herniations occur in
the cervical region (C2-C7) with 80% of these at C2-3. In the thoracolumbar
spinal column, 80% of the IVD herniations occur with 67-75% of these occurring
at T12-13 or T13-L1. The incidence rapidly dissipates cranially and caudally
from the TL junction. The incidence between T1 and T9 is less than 0.5%.
From L4 caudally, each disc has an incidence of around 2.5%. Cervical IVD
herniation will cause quadriparesis (or quadriplegia) while TL IVD herniations
result in paraparesis to paraplegia.
 In addition to
location, the dynamic factor dictates the severity of clinical signs. The
amount of traumatic force imparted by a small amount of material traveling
rapidly is greater than a larger amount going slow. In the worst case,
this means the time for intervention is also quiet short. In most cases
of IVD disease, definitive treatment must be started before 24 hours in
order to achieve the greatest success. In some cases, this time is shorter.
Unfortunately, delaying treatment to see the outcome may preclude success.
We treat severe IVD disease as a medical and surgical emergency. In patients
with complete motor and sensory paralysis, the patient should be treated
for acute spinal injury and be immediately referred to a center who can
diagnose and definitively treat the problem. In patients who are paralyzed
but retain deep pain, then it is possible to treat them for acute spinal
injury and observe them for signs of improvement. If they are worse or
no better within 24 hours, they then constitute and emergency referral.
On the other hand, it is best to refer these patients at the outset. In
patients with mild paresis or mere back pain, they can be worked-up for
the rule/out and referred if they do not make improvements in 5-7 days.
These later patients may benefit from surgical intervention, but might
also recover from the current IVD herniation with medical management. They
are still surgical candidates upon recovery, to prevent future IVD disease.
In addition to
location, the dynamic factor dictates the severity of clinical signs. The
amount of traumatic force imparted by a small amount of material traveling
rapidly is greater than a larger amount going slow. In the worst case,
this means the time for intervention is also quiet short. In most cases
of IVD disease, definitive treatment must be started before 24 hours in
order to achieve the greatest success. In some cases, this time is shorter.
Unfortunately, delaying treatment to see the outcome may preclude success.
We treat severe IVD disease as a medical and surgical emergency. In patients
with complete motor and sensory paralysis, the patient should be treated
for acute spinal injury and be immediately referred to a center who can
diagnose and definitively treat the problem. In patients who are paralyzed
but retain deep pain, then it is possible to treat them for acute spinal
injury and observe them for signs of improvement. If they are worse or
no better within 24 hours, they then constitute and emergency referral.
On the other hand, it is best to refer these patients at the outset. In
patients with mild paresis or mere back pain, they can be worked-up for
the rule/out and referred if they do not make improvements in 5-7 days.
These later patients may benefit from surgical intervention, but might
also recover from the current IVD herniation with medical management. They
are still surgical candidates upon recovery, to prevent future IVD disease.
Medical management of IVD disease consists of absolute rest for a minimum
of 30 days or 3 weeks beyond return to clinical normalcy. This confinement
must be in a cage no more than 2.5 x 1.5 times the animal's body length.
An airline carrier is ideal. Many patients will benefit from corticosteroid
management during the initiation of treatment. I think this should only
be done under direct veterinary supervision. If the patient feels better
and then becomes active before healing has occurred, they are at great
risk to get worse. We see this outcome commonly. It could be prevented
in many cases, with absolute confinement of the patient. Owners do not
always comply, allowing their pet to worsen. For that reason, I prefer
to treat these patients in the hospital for the first 5-7 days, going home
without medication, only confinement. I would give 30 mg/kg of methylprednisolone
(Solu Medral or Solu Delta Cortef) IV, initially; followed by 15 mg/kg
every 8 hours for the first 24 hours. Then, I give oral prednisolone at
1 mg/kg/day in 2 divided doses for 5 days. If more steroids are needed,
I give 0.5 mg/kg every other day in the morning. During steroid medication,
it is necessary to protect against steroid-gastritis. I use misoprostil
(50-100 µg) every 12 hours until using alternate day steroids. Many
patients feel better with muscle relaxants. I prefer diazepam at 0.25-0.5
mg/kg every 8 hours. Once the animal has recovered and has been normal
without medication for 3 weeks, prophylactic IVD fenestration can be performed.
It is felt that 60% of patients with moderate to mild IVD disease will
recover with medical management. On the other 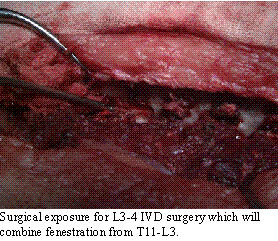 hand,
50-80% of these patients will experience additional IVD disease at the
same or other site during their lives. Clinically, I usually see recurrence
of IVD disease in patients without prophylactic fenestration every 6 months
to a year.
hand,
50-80% of these patients will experience additional IVD disease at the
same or other site during their lives. Clinically, I usually see recurrence
of IVD disease in patients without prophylactic fenestration every 6 months
to a year.
 As such, I prefer
the surgical approach, decompression to treat the acute disease combined
with fenestration to prevent future problems. Fenestration is a statistical
game, removing the nuclear material (and creating fibrosis within the disc
for additional support) so that the chance of IVD herniation at the fenestrated
site is lessened. Fenestration does not remove material from the neural
canal, laminectomy is needed for that. In the neck, fenestration of C2-C6
reduces the likelihood of future herniation by 99% in that region (.99
x .2, overall). In the TL region, fenestration of T11-L3 reduces the chances
by 95% in that region (.92 x .8, overall). By combining cervical and TL
fenestration, the overall chances of recurrent IVD disease is reduced by
93%. While not all patients read the same statistical books, generally
this will eliminate future IVD disease. If decompression is needed for
the patient to recover, fenestration can be performed to prevent recurrence.
In cases where fenestration has not been done, the patient remains at risk
for recurrent IVD disease.
As such, I prefer
the surgical approach, decompression to treat the acute disease combined
with fenestration to prevent future problems. Fenestration is a statistical
game, removing the nuclear material (and creating fibrosis within the disc
for additional support) so that the chance of IVD herniation at the fenestrated
site is lessened. Fenestration does not remove material from the neural
canal, laminectomy is needed for that. In the neck, fenestration of C2-C6
reduces the likelihood of future herniation by 99% in that region (.99
x .2, overall). In the TL region, fenestration of T11-L3 reduces the chances
by 95% in that region (.92 x .8, overall). By combining cervical and TL
fenestration, the overall chances of recurrent IVD disease is reduced by
93%. While not all patients read the same statistical books, generally
this will eliminate future IVD disease. If decompression is needed for
the patient to recover, fenestration can be performed to prevent recurrence.
In cases where fenestration has not been done, the patient remains at risk
for recurrent IVD disease.
 The diagnosis
of IVD disease is made with radiographs and myelography. Since many cases
present with acute signs, EMG does not offer assistance. In some cases,
CSF analysis helps rule/out meningomyelitis, but myelography is what determines
the extent and surgical approach of choice. In most cases, this will be
hemilaminectomy. Myelography helps to confirm the side upon which to perform
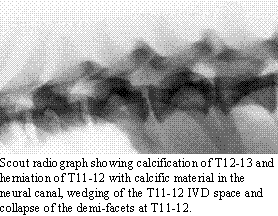
the laminectomy. Scout radiographs may demonstrate the presence of degenerative
disc disease by revealing calcified nuclear material. The site of herniation
may show collapse of the IVD space, wedging of the IVD space, collapse
of the demi-facets and the presence of calcified material in the neural
canal. Coupled with the neurologic examination, this may be enough to determine
the need for surgical intervention. When there is doubt about the location
or the radiographic changes do not fit the neurologic findings, myelography
is needed. Myelography will also help rule/out other diseases which might
cause spinal cord compression, such as
The diagnosis
of IVD disease is made with radiographs and myelography. Since many cases
present with acute signs, EMG does not offer assistance. In some cases,
CSF analysis helps rule/out meningomyelitis, but myelography is what determines
the extent and surgical approach of choice. In most cases, this will be
hemilaminectomy. Myelography helps to confirm the side upon which to perform
the laminectomy. Scout radiographs may demonstrate the presence of degenerative
disc disease by revealing calcified nuclear material. The site of herniation
may show collapse of the IVD space, wedging of the IVD space, collapse
of the demi-facets and the presence of calcified material in the neural
canal. Coupled with the neurologic examination, this may be enough to determine
the need for surgical intervention. When there is doubt about the location
or the radiographic changes do not fit the neurologic findings, myelography
is needed. Myelography will also help rule/out other diseases which might
cause spinal cord compression, such as 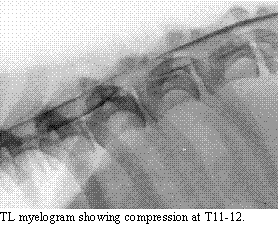 neoplasia.
neoplasia.
 Beyond treatment
and surgical fenestration, it is possible that certain dietary supplements
would benefit chondrodystrophic patients to prevent IVD disease or to facilitate
their recovery upon IVD herniation. Tofu (rich in soy lecithin) may aid
in spinal cord myelination. Antioxidants like vitamin E, vitamin C and
ginkgo biloba may help prevent degeneration and, certainly, appear to help
protect the spinal cord from the results of spinal cord injury. Vitamin
E and C must be given prior to the damage, while ginkgo biloba may be as
effective as methylprednisolone in treating the injury once it has happened.
Our current understanding of spinal cord damage suggests that antioxidants
may work by sparing spinal cord function, while the steroid receptor may
help protect spinal cord architecture. As such, methylprednisolone, which
contains both the antioxidant effect and steroid receptor effect, is currently
the best medication for the treatment of brain and spinal cord injury.
Beyond treatment
and surgical fenestration, it is possible that certain dietary supplements
would benefit chondrodystrophic patients to prevent IVD disease or to facilitate
their recovery upon IVD herniation. Tofu (rich in soy lecithin) may aid
in spinal cord myelination. Antioxidants like vitamin E, vitamin C and
ginkgo biloba may help prevent degeneration and, certainly, appear to help
protect the spinal cord from the results of spinal cord injury. Vitamin
E and C must be given prior to the damage, while ginkgo biloba may be as
effective as methylprednisolone in treating the injury once it has happened.
Our current understanding of spinal cord damage suggests that antioxidants
may work by sparing spinal cord function, while the steroid receptor may
help protect spinal cord architecture. As such, methylprednisolone, which
contains both the antioxidant effect and steroid receptor effect, is currently
the best medication for the treatment of brain and spinal cord injury.
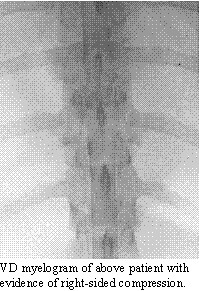
Fibrocartilaginous Infarction:
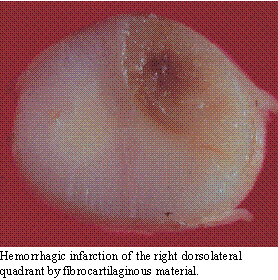 Even though animals
do not suffer from the same degree of vascular disease as human beings,
infarction of the spinal cord with fibrocartilaginous material is not uncommon.
It occurs in any breed of dogs, but is most common in large breeds, such
as Great Danes, Labrador retrievers and German Shepherds. Although both
arteries and veins can be affected, most commonly it is the venous system
of the spinal cord which is obstructed, leading to a hemorrhagic infarction.
It is believed that herniation of the nucleus pulposus takes place either
into the vertebral body or the venous sinuses within the spinal column.
Since the vertebral body represents a vascular space communicating with
the spinal venous system, the material gains access to the spinal veins.
These veins do not have valves, allowing the fibrocartilaginous material
to flow up and down the spinal column. When intra-thoracic pressure increases,
this material can be back-flushed into small penetrating spinal cord veins.
When the intra-thoracic pressure returns to normal, the veins collapse
trapping the material and leading to excessive venous pressure upstream
to the occlusion. The venules rupture leading to a hemorrhagic infarction.
The pattern of infarction usually affects a quadrant of the spinal cord,
although initial signs may affect more of the spinal pathways due to inflammation
and spinal cord swelling. The infarction can occur anywhere along the spinal
cord, but the causal cervical and mid- to lower lumbar spinal cord segments
appear to be most frequently involved.
Even though animals
do not suffer from the same degree of vascular disease as human beings,
infarction of the spinal cord with fibrocartilaginous material is not uncommon.
It occurs in any breed of dogs, but is most common in large breeds, such
as Great Danes, Labrador retrievers and German Shepherds. Although both
arteries and veins can be affected, most commonly it is the venous system
of the spinal cord which is obstructed, leading to a hemorrhagic infarction.
It is believed that herniation of the nucleus pulposus takes place either
into the vertebral body or the venous sinuses within the spinal column.
Since the vertebral body represents a vascular space communicating with
the spinal venous system, the material gains access to the spinal veins.
These veins do not have valves, allowing the fibrocartilaginous material
to flow up and down the spinal column. When intra-thoracic pressure increases,
this material can be back-flushed into small penetrating spinal cord veins.
When the intra-thoracic pressure returns to normal, the veins collapse
trapping the material and leading to excessive venous pressure upstream
to the occlusion. The venules rupture leading to a hemorrhagic infarction.
The pattern of infarction usually affects a quadrant of the spinal cord,
although initial signs may affect more of the spinal pathways due to inflammation
and spinal cord swelling. The infarction can occur anywhere along the spinal
cord, but the causal cervical and mid- to lower lumbar spinal cord segments
appear to be most frequently involved.
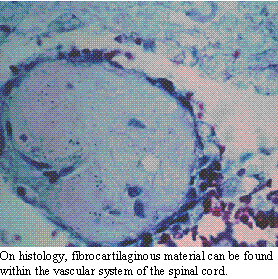 The presence
of spinal cord infarction should be suspected whenever a patient presents
with acute onset of paresis or paralysis which is markedly asymmetrical
and there is no evidence of hyperpathia. Vascular disease is generally
acute and non-progressive. In addition, the spinal cord contains pain pathways,
but no pain receptors. As such, strict diseases within the spinal cord
without meningeal involvement are usually not painful. Most of the other
diagnostic tests will be within normal limits. Occasionally, there will
be evidence of hemorrhage on CSF analysis. Spinal radiographs, do not demonstrate
the disease, but may reveal other evidence of spinal column degeneration.
Myelography will be normal or demonstrate mild intramedullary swelling.
In a small number of cases (where the vascular occlusion is secondary to
a systemic disease), the minimum data base will show evidence of the systemic
disease.
The presence
of spinal cord infarction should be suspected whenever a patient presents
with acute onset of paresis or paralysis which is markedly asymmetrical
and there is no evidence of hyperpathia. Vascular disease is generally
acute and non-progressive. In addition, the spinal cord contains pain pathways,
but no pain receptors. As such, strict diseases within the spinal cord
without meningeal involvement are usually not painful. Most of the other
diagnostic tests will be within normal limits. Occasionally, there will
be evidence of hemorrhage on CSF analysis. Spinal radiographs, do not demonstrate
the disease, but may reveal other evidence of spinal column degeneration.
Myelography will be normal or demonstrate mild intramedullary swelling.
In a small number of cases (where the vascular occlusion is secondary to
a systemic disease), the minimum data base will show evidence of the systemic
disease.
The treatment of spinal cord infarction is that for acute spinal cord
injury, using methylprednisolone at 30 mg/kg initially. This is followed
by 15 mg/kg every 8 hours for the first 24-48 hours. Then, oral prednisolone
is begun at 0.5 mg /kg every 12 hours for 5 days. I continue prednisolone
at 0.5 mg/kg every other day, in the morning, for up to another 2 weeks.
Many cases will improve dramatically within the first week, although they
will still improve over several months. If there has been no improvement
in the first week, re-examination and additional tests may be indicated.
Since usually only a quadrant of the spinal cord is affected, the patient
will improve most on the unaffected side. Reorganization will usually allow
these patients to function adequately. Spinal cord infarction from fibrocartilaginous
material is a sporadic problem and, usually, does not reoccur.
Copyright Dog2Doc.com 1997
All Rights Reserved
 Hop back to the Dog2Doc Home Page!
Hop back to the Dog2Doc Home Page!
Last updated 27 August 2002
 On the other hand,
chondrodystrophic breeds of dogs are prone to the development of IVD herniation
early in life. In these breeds (including dachshunds, beagles, pekinese,
miniature poodles, cocker spaniels, pomeranians and basset hounds), there
is a metaplasia of the nucleus pulposus whereby the normal collagen fibers
of the nucleus are replaced by hyaline fibers. The hyaline fibers are less
elastic than collagen fibers leading to degeneration of the annulus fibrosis.
The hyaline fibers during this degenerative process calcify, creating further
inelasticity. Due to the fact that the annulus fibrosis is thinnest dorsally
toward the spinal cord, the least line of resistance for the degeneration
and breakdown of the annulus is toward the spinal cord. Ultimately, the
annulus ruptures allowing the herniation of the degenerative nucleus into
the neural canal, compressing the spinal cord. Not only does the IVD material
compress the spinal cord, but the degenerative material is irritative in
nature. The presence of the herniated material in the epidural space causes
inflammation, furthering the swelling associated with the herniation.
On the other hand,
chondrodystrophic breeds of dogs are prone to the development of IVD herniation
early in life. In these breeds (including dachshunds, beagles, pekinese,
miniature poodles, cocker spaniels, pomeranians and basset hounds), there
is a metaplasia of the nucleus pulposus whereby the normal collagen fibers
of the nucleus are replaced by hyaline fibers. The hyaline fibers are less
elastic than collagen fibers leading to degeneration of the annulus fibrosis.
The hyaline fibers during this degenerative process calcify, creating further
inelasticity. Due to the fact that the annulus fibrosis is thinnest dorsally
toward the spinal cord, the least line of resistance for the degeneration
and breakdown of the annulus is toward the spinal cord. Ultimately, the
annulus ruptures allowing the herniation of the degenerative nucleus into
the neural canal, compressing the spinal cord. Not only does the IVD material
compress the spinal cord, but the degenerative material is irritative in
nature. The presence of the herniated material in the epidural space causes
inflammation, furthering the swelling associated with the herniation.
 The neurologic
assessment of patients with rear leg problems helps to confirm that the
disease is neurologic in nature and its location. Weakness can indicate
neurologic disease, muscle disease or systemic illness. On the other hand,
reproducible deficits in proprioception usually is indicative of neurologic
disease, whether knuckling, stumbling or falling or conscious proprioceptive
deficits or dysmetria of unconscious proprioceptive dysfunction. When deciding
whether a rear leg lameness is secondary to orthopedic or neurologic disease,
examination of proprioceptive function can help make the differentiation.
The neurologic
assessment of patients with rear leg problems helps to confirm that the
disease is neurologic in nature and its location. Weakness can indicate
neurologic disease, muscle disease or systemic illness. On the other hand,
reproducible deficits in proprioception usually is indicative of neurologic
disease, whether knuckling, stumbling or falling or conscious proprioceptive
deficits or dysmetria of unconscious proprioceptive dysfunction. When deciding
whether a rear leg lameness is secondary to orthopedic or neurologic disease,
examination of proprioceptive function can help make the differentiation.
 In addition to
location, the dynamic factor dictates the severity of clinical signs. The
amount of traumatic force imparted by a small amount of material traveling
rapidly is greater than a larger amount going slow. In the worst case,
this means the time for intervention is also quiet short. In most cases
of IVD disease, definitive treatment must be started before 24 hours in
order to achieve the greatest success. In some cases, this time is shorter.
Unfortunately, delaying treatment to see the outcome may preclude success.
We treat severe IVD disease as a medical and surgical emergency. In patients
with complete motor and sensory paralysis, the patient should be treated
for acute spinal injury and be immediately referred to a center who can
diagnose and definitively treat the problem. In patients who are paralyzed
but retain deep pain, then it is possible to treat them for acute spinal
injury and observe them for signs of improvement. If they are worse or
no better within 24 hours, they then constitute and emergency referral.
On the other hand, it is best to refer these patients at the outset. In
patients with mild paresis or mere back pain, they can be worked-up for
the rule/out and referred if they do not make improvements in 5-7 days.
These later patients may benefit from surgical intervention, but might
also recover from the current IVD herniation with medical management. They
are still surgical candidates upon recovery, to prevent future IVD disease.
In addition to
location, the dynamic factor dictates the severity of clinical signs. The
amount of traumatic force imparted by a small amount of material traveling
rapidly is greater than a larger amount going slow. In the worst case,
this means the time for intervention is also quiet short. In most cases
of IVD disease, definitive treatment must be started before 24 hours in
order to achieve the greatest success. In some cases, this time is shorter.
Unfortunately, delaying treatment to see the outcome may preclude success.
We treat severe IVD disease as a medical and surgical emergency. In patients
with complete motor and sensory paralysis, the patient should be treated
for acute spinal injury and be immediately referred to a center who can
diagnose and definitively treat the problem. In patients who are paralyzed
but retain deep pain, then it is possible to treat them for acute spinal
injury and observe them for signs of improvement. If they are worse or
no better within 24 hours, they then constitute and emergency referral.
On the other hand, it is best to refer these patients at the outset. In
patients with mild paresis or mere back pain, they can be worked-up for
the rule/out and referred if they do not make improvements in 5-7 days.
These later patients may benefit from surgical intervention, but might
also recover from the current IVD herniation with medical management. They
are still surgical candidates upon recovery, to prevent future IVD disease.
 hand,
50-80% of these patients will experience additional IVD disease at the
same or other site during their lives. Clinically, I usually see recurrence
of IVD disease in patients without prophylactic fenestration every 6 months
to a year.
hand,
50-80% of these patients will experience additional IVD disease at the
same or other site during their lives. Clinically, I usually see recurrence
of IVD disease in patients without prophylactic fenestration every 6 months
to a year.
 As such, I prefer
the surgical approach, decompression to treat the acute disease combined
with fenestration to prevent future problems. Fenestration is a statistical
game, removing the nuclear material (and creating fibrosis within the disc
for additional support) so that the chance of IVD herniation at the fenestrated
site is lessened. Fenestration does not remove material from the neural
canal, laminectomy is needed for that. In the neck, fenestration of C2-C6
reduces the likelihood of future herniation by 99% in that region (.99
x .2, overall). In the TL region, fenestration of T11-L3 reduces the chances
by 95% in that region (.92 x .8, overall). By combining cervical and TL
fenestration, the overall chances of recurrent IVD disease is reduced by
93%. While not all patients read the same statistical books, generally
this will eliminate future IVD disease. If decompression is needed for
the patient to recover, fenestration can be performed to prevent recurrence.
In cases where fenestration has not been done, the patient remains at risk
for recurrent IVD disease.
As such, I prefer
the surgical approach, decompression to treat the acute disease combined
with fenestration to prevent future problems. Fenestration is a statistical
game, removing the nuclear material (and creating fibrosis within the disc
for additional support) so that the chance of IVD herniation at the fenestrated
site is lessened. Fenestration does not remove material from the neural
canal, laminectomy is needed for that. In the neck, fenestration of C2-C6
reduces the likelihood of future herniation by 99% in that region (.99
x .2, overall). In the TL region, fenestration of T11-L3 reduces the chances
by 95% in that region (.92 x .8, overall). By combining cervical and TL
fenestration, the overall chances of recurrent IVD disease is reduced by
93%. While not all patients read the same statistical books, generally
this will eliminate future IVD disease. If decompression is needed for
the patient to recover, fenestration can be performed to prevent recurrence.
In cases where fenestration has not been done, the patient remains at risk
for recurrent IVD disease.
 The diagnosis
of IVD disease is made with radiographs and myelography. Since many cases
present with acute signs, EMG does not offer assistance. In some cases,
CSF analysis helps rule/out meningomyelitis, but myelography is what determines
the extent and surgical approach of choice. In most cases, this will be
hemilaminectomy. Myelography helps to confirm the side upon which to perform
the laminectomy. Scout radiographs may demonstrate the presence of degenerative
disc disease by revealing calcified nuclear material. The site of herniation
may show collapse of the IVD space, wedging of the IVD space, collapse
of the demi-facets and the presence of calcified material in the neural
canal. Coupled with the neurologic examination, this may be enough to determine
the need for surgical intervention. When there is doubt about the location
or the radiographic changes do not fit the neurologic findings, myelography
is needed. Myelography will also help rule/out other diseases which might
cause spinal cord compression, such as
The diagnosis
of IVD disease is made with radiographs and myelography. Since many cases
present with acute signs, EMG does not offer assistance. In some cases,
CSF analysis helps rule/out meningomyelitis, but myelography is what determines
the extent and surgical approach of choice. In most cases, this will be
hemilaminectomy. Myelography helps to confirm the side upon which to perform
the laminectomy. Scout radiographs may demonstrate the presence of degenerative
disc disease by revealing calcified nuclear material. The site of herniation
may show collapse of the IVD space, wedging of the IVD space, collapse
of the demi-facets and the presence of calcified material in the neural
canal. Coupled with the neurologic examination, this may be enough to determine
the need for surgical intervention. When there is doubt about the location
or the radiographic changes do not fit the neurologic findings, myelography
is needed. Myelography will also help rule/out other diseases which might
cause spinal cord compression, such as  neoplasia.
neoplasia.
 Beyond treatment
and surgical fenestration, it is possible that certain dietary supplements
would benefit chondrodystrophic patients to prevent IVD disease or to facilitate
their recovery upon IVD herniation. Tofu (rich in soy lecithin) may aid
in spinal cord myelination. Antioxidants like vitamin E, vitamin C and
ginkgo biloba may help prevent degeneration and, certainly, appear to help
protect the spinal cord from the results of spinal cord injury. Vitamin
E and C must be given prior to the damage, while ginkgo biloba may be as
effective as methylprednisolone in treating the injury once it has happened.
Our current understanding of spinal cord damage suggests that antioxidants
may work by sparing spinal cord function, while the steroid receptor may
help protect spinal cord architecture. As such, methylprednisolone, which
contains both the antioxidant effect and steroid receptor effect, is currently
the best medication for the treatment of brain and spinal cord injury.
Beyond treatment
and surgical fenestration, it is possible that certain dietary supplements
would benefit chondrodystrophic patients to prevent IVD disease or to facilitate
their recovery upon IVD herniation. Tofu (rich in soy lecithin) may aid
in spinal cord myelination. Antioxidants like vitamin E, vitamin C and
ginkgo biloba may help prevent degeneration and, certainly, appear to help
protect the spinal cord from the results of spinal cord injury. Vitamin
E and C must be given prior to the damage, while ginkgo biloba may be as
effective as methylprednisolone in treating the injury once it has happened.
Our current understanding of spinal cord damage suggests that antioxidants
may work by sparing spinal cord function, while the steroid receptor may
help protect spinal cord architecture. As such, methylprednisolone, which
contains both the antioxidant effect and steroid receptor effect, is currently
the best medication for the treatment of brain and spinal cord injury.
 Even though animals
do not suffer from the same degree of vascular disease as human beings,
infarction of the spinal cord with fibrocartilaginous material is not uncommon.
It occurs in any breed of dogs, but is most common in large breeds, such
as Great Danes, Labrador retrievers and German Shepherds. Although both
arteries and veins can be affected, most commonly it is the venous system
of the spinal cord which is obstructed, leading to a hemorrhagic infarction.
It is believed that herniation of the nucleus pulposus takes place either
into the vertebral body or the venous sinuses within the spinal column.
Since the vertebral body represents a vascular space communicating with
the spinal venous system, the material gains access to the spinal veins.
These veins do not have valves, allowing the fibrocartilaginous material
to flow up and down the spinal column. When intra-thoracic pressure increases,
this material can be back-flushed into small penetrating spinal cord veins.
When the intra-thoracic pressure returns to normal, the veins collapse
trapping the material and leading to excessive venous pressure upstream
to the occlusion. The venules rupture leading to a hemorrhagic infarction.
The pattern of infarction usually affects a quadrant of the spinal cord,
although initial signs may affect more of the spinal pathways due to inflammation
and spinal cord swelling. The infarction can occur anywhere along the spinal
cord, but the causal cervical and mid- to lower lumbar spinal cord segments
appear to be most frequently involved.
Even though animals
do not suffer from the same degree of vascular disease as human beings,
infarction of the spinal cord with fibrocartilaginous material is not uncommon.
It occurs in any breed of dogs, but is most common in large breeds, such
as Great Danes, Labrador retrievers and German Shepherds. Although both
arteries and veins can be affected, most commonly it is the venous system
of the spinal cord which is obstructed, leading to a hemorrhagic infarction.
It is believed that herniation of the nucleus pulposus takes place either
into the vertebral body or the venous sinuses within the spinal column.
Since the vertebral body represents a vascular space communicating with
the spinal venous system, the material gains access to the spinal veins.
These veins do not have valves, allowing the fibrocartilaginous material
to flow up and down the spinal column. When intra-thoracic pressure increases,
this material can be back-flushed into small penetrating spinal cord veins.
When the intra-thoracic pressure returns to normal, the veins collapse
trapping the material and leading to excessive venous pressure upstream
to the occlusion. The venules rupture leading to a hemorrhagic infarction.
The pattern of infarction usually affects a quadrant of the spinal cord,
although initial signs may affect more of the spinal pathways due to inflammation
and spinal cord swelling. The infarction can occur anywhere along the spinal
cord, but the causal cervical and mid- to lower lumbar spinal cord segments
appear to be most frequently involved.
 The presence
of spinal cord infarction should be suspected whenever a patient presents
with acute onset of paresis or paralysis which is markedly asymmetrical
and there is no evidence of hyperpathia. Vascular disease is generally
acute and non-progressive. In addition, the spinal cord contains pain pathways,
but no pain receptors. As such, strict diseases within the spinal cord
without meningeal involvement are usually not painful. Most of the other
diagnostic tests will be within normal limits. Occasionally, there will
be evidence of hemorrhage on CSF analysis. Spinal radiographs, do not demonstrate
the disease, but may reveal other evidence of spinal column degeneration.
Myelography will be normal or demonstrate mild intramedullary swelling.
In a small number of cases (where the vascular occlusion is secondary to
a systemic disease), the minimum data base will show evidence of the systemic
disease.
The presence
of spinal cord infarction should be suspected whenever a patient presents
with acute onset of paresis or paralysis which is markedly asymmetrical
and there is no evidence of hyperpathia. Vascular disease is generally
acute and non-progressive. In addition, the spinal cord contains pain pathways,
but no pain receptors. As such, strict diseases within the spinal cord
without meningeal involvement are usually not painful. Most of the other
diagnostic tests will be within normal limits. Occasionally, there will
be evidence of hemorrhage on CSF analysis. Spinal radiographs, do not demonstrate
the disease, but may reveal other evidence of spinal column degeneration.
Myelography will be normal or demonstrate mild intramedullary swelling.
In a small number of cases (where the vascular occlusion is secondary to
a systemic disease), the minimum data base will show evidence of the systemic
disease.